Introduction:
Children with sickle cell disease (SCD) often experience frequent severe acute vaso-occlusive pain events, unresponsive to FDA-approved therapies (hydroxyurea, L-glutamine, crizanlizumab) to decrease the severe acute vaso-occlusive pain event incidence rate. The ASH guidelines for pain management recommend regular blood transfusion therapy after FDA-approved therapies have failed to decrease the high rate of severe acute vaso-occlusive pain events ( The Journal of Pediatrics, 139(6), 785-789). However, unlike regular blood transfusion therapy for primary and secondary stroke prevention, for which the goal hemoglobin S (Hb S) level is ≤ 30% , no optimal Hb S threshold has been established to attenuate the severe acute vaso-occlusive pain event incidence rate. We tested the hypothesis that among children receiving regular blood transfusion therapy with a goal to keep the mean hemoglobin S level < 30%, participants that achieved a mean Hb S level < 30% would have lower rates of severe acute vaso-occlusive pain events requiring hospitalization than children regularly transfused with mean Hb S level > 30%. Evidence to support our hypothesis would provide the first evidence-based guidelines for the optimal hemoglobin S threshold to decrease the acute vaso-occlusive pain event incidence rate in children with sickle cell anemia (HbSS).
Methods:
The SIT trial was a single-blind clinical trial( Blood Advances, 4(12), 2656-2701), where participants were children with sickle cell anemia randomly allocated to receive regular blood transfusion therapy (transfusion group) or standard care (observation group). Participants receiving regular blood transfusion therapy from the SIT Trial were included in the secondary analysis. Eligibility criteria include participants between 5 and 15 years of age, with no history of stroke, one or more silent cerebral infarcts on magnetic resonance imaging, and a neurologic examination showing no abnormalities. The transfusion therapy goal was to keep the maximum Hb S level < 30%. For the ancillary study, participants were included only in the transfusion arm of the trial. The primary endpoint of this ancillary study was the incidence rate of hospitalization for vaso-occlusive pain episodes among those receiving only regular blood transfusion therapy. We conducted a zero-inflated negative binomial regression analysis to determine the association between the incidence rate for severe acute vaso-occlusive pain events requiring hospitalization and independent covariates, including age at the time of random allocation, sex, and mean hemoglobin S level (< 30% and > 30%).
Results:
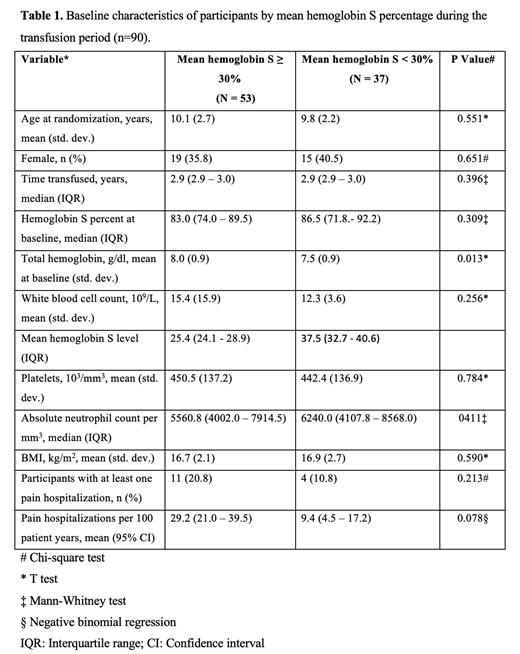
A total of 90 participants received regular blood transfusion therapy in the SIT trial. Among participants transfused, 53 and 37 individuals had a mean Hb S level of < 30% and > 30%, respectively. The median time on transfusions for the groups with Hb S levels of < 30% and > 30% was 2.9 years for both groups. Among the participants, 10.8% (4/37) who had a mean Hb S < 30% and 20.8% (11/53) of those with a mean Hb S > 30% experienced at least one hospitalization for a severe acute vaso-occlusive pain event. The mean hemoglobin S levels in the < 30% and > 30% groups were 25.4% and 37.5%, respectively. The mean hospital rates for severe acute vaso-occlusive pain events in the two groups < 30% and >30% were 9.4 (95% confidence interval 9.4 - 17.2 person-years) and 29.2 per 100 person-years (95% confidence interval, 21.0 - 39.5 person-years), respectively, P = 0.078. In the multivariable analysis, the incidence rate ratio for severe acute vaso-occlusive pain events showed that there was no statistical difference between those with a mean Hb S level in groups < 30% and > 30%, 0.65; P=0.43. Children with a higher baseline hemoglobin level were more likely to achieve a mean hemoglobin S level < 30%.
Conclusion: In children with sickle cell anemia, receiving regular blood transfusion therapy with the goal of keeping the mean hemoglobin S level < 30%, achieving a mean hemoglobin S level > 30% was as effective as a mean hemoglobin S level < 30 % in decreasing the incidence rate of severe acute vaso-occlusive pain events requiring hospitalization. Further studies are warranted to identify reliable measures of the efficacy of regular blood transfusion therapy for the reduction of acute vaso-occlusive pain events.
Disclosures
DeBaun:Vertex/CRISPR: Membership on an entity's Board of Directors or advisory committees; Novartis Pharmaceuticals Corporation: Other: Study steering committee member; Global Blood Therapeutics: Membership on an entity's Board of Directors or advisory committees, Research Funding; FORMA: Consultancy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal